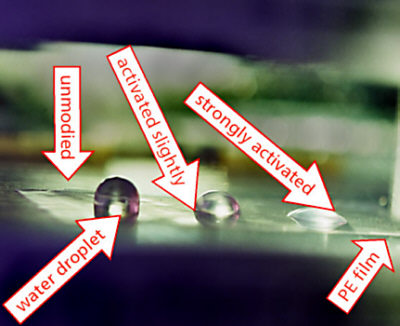
The shape of the water drops on a solid is determined by a few layers of atoms at the surface. It is a region which is about 1 nm thick. At a polyethylene film the drop is almost a hemisphere. The contact angle is about 100°. The oxidation of a shallow surface layer of this film will make the surface more wettable and reduce the contact angle.
This process is referred to as activation and it can considerably improve the material's properties.
Technical surface activation treatments of polymers result in a complex mixture of functional groups. For many applications (e.g. printing on plastic bags, adhesive joining of polyolefins) this mixture does the job very well and the exact type of functional groups is not important.
If a defined function is required, the functional groups formed in the activation process (-OH, C=O, COOH and others) can be used as anchor groups for more specific reactions. The combination of activation and organic chemistry allows the efficient production of surfaces with well-defined chemical properties. Coupling oligomers and polymers, for example, is a very versatile way of a surface functionalization.
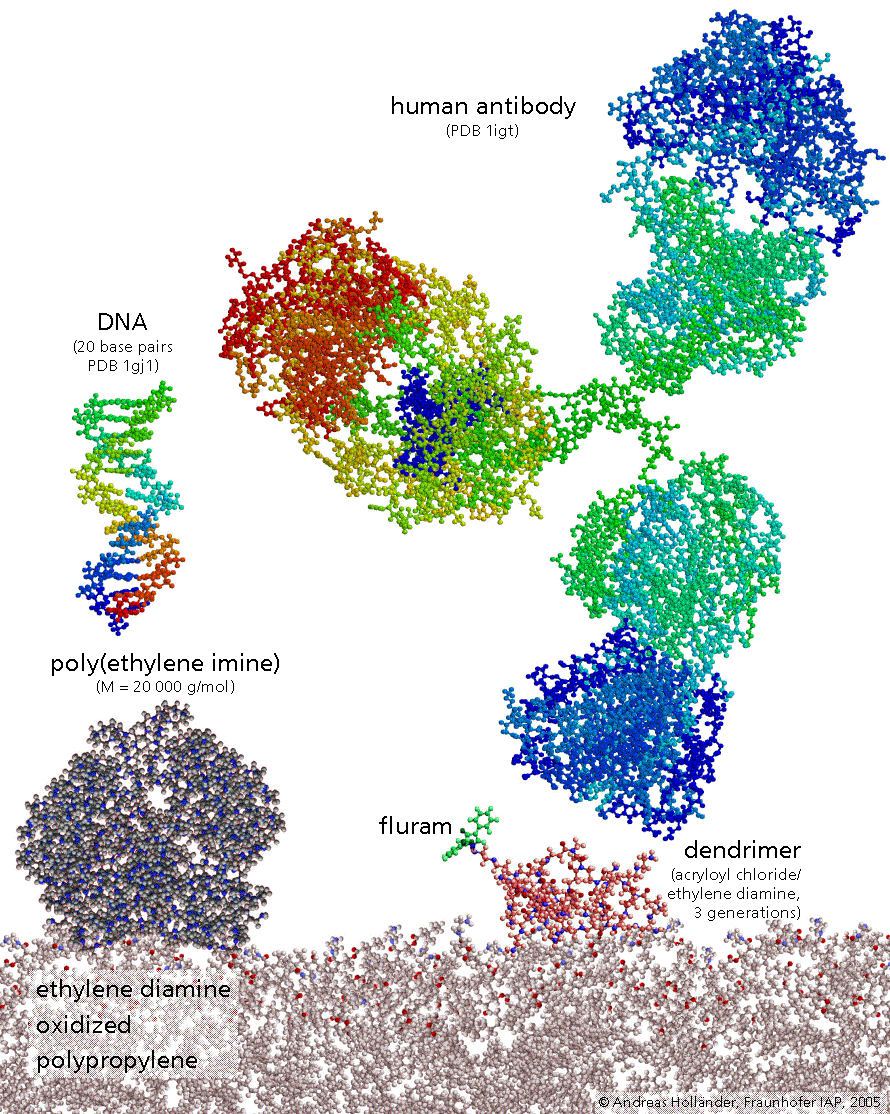
The image shows a functionalized polymer surface on a molecular scale. It is a polypropylene surface which was oxidized (red dots are oxygen atoms) and then reacted with a diamine (blue dots are nitrogen atoms). For comparison there are also shown in scale: DNA fragment, poly(ethylene imine), a dedrimer, and an antibody.