Why use VUV lamps
UV light with a wavelength < 200 nm can break chemical bonds in many organic materials. This way it generates reactive radicals without a dedicated initiator. Consequently, it can initiate efficient reactions without a specific catalyst.
The advantages:
- cost reduction
- less usage of valuable resources
- new fields of application due to avoiding harmful chemicals (e.g. food contact)
For more details about the technology please see here.
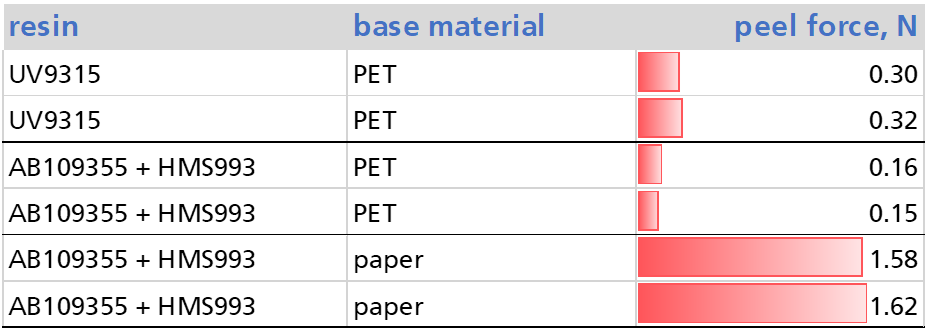
Silicone resins
There are basically two types of silicone reactive resins. Thermally curing resins rely on the reaction of silane Si-H bonds with C=C double bonds to form a Si-C bond. For a sufficiently fast reaction catalysts are added which contain a metal of the platinum group.
The second type of silicone resins contains organic functional groups which react with each other to form the crosslinks during the curing process. This can be, for example, epoxides which undergo reactions initiated by photo initiator absorbing light typically in the UV A of B range.
With VUV curing, neither a catalyst nor an initiator is necessary.