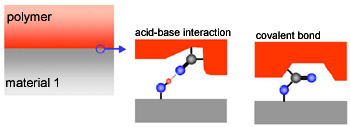
Adhesion
Adhesive interactions are based on a number of different effects. Mechanical interlocking is not important on very flat joints. The interface mixing differs in its importance depending on the materials to be joint. And there are chemical interactions (figure): Acid-base interactions (= electron donor-acceptor interactions) are rather weak but they can be numerous. Chemical bonds are the strongest interactions but they are also quite demanding with respect to the conditions of their formation. The right partners must meet with a precise spatial orientation.
Bonding plastics
Considering the chemical interactions, it seems to be feasible to join two flat surfaces if they are brought into molecular contact and if there are appropriate chemical structures on the interface.
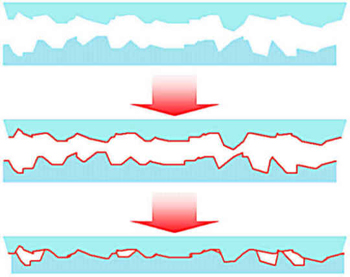
However, there are very few interactions between the surfaces of usual polymers (upper part of the picture). A surface treatment creates interaction sites (red line in the picture). If they are brought into molecular contact interactions take place which can be recorded as an adhesion force (lower picture).